

Mechanism of antioxidants
1. Lipid Oxidation
The overall mechanism of lipid oxidation consists of three phases:
(1) Initiation, the formation of free radicals;
RH + O2 -->R· + ·OH
R· + O2 --> · + ROO·
(2) Propagation, the free-radical chain reactions;
ROO· + RH --> R· + ROOH
ROOH--> RO· + HO·
(3) Termination, the formation of nonradical products.
R· + R· --> RR
R· + ROO·--> ROOR
ROO· + ROO· --> ROOR + O2
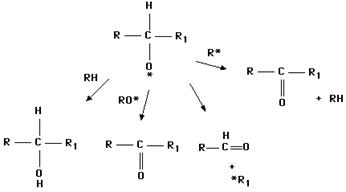
This reaction scheme is capable of generating aldehydes, ketones, alcohols and hydrocarbons. Many of the volatile compounds formed during lipid oxidation originate through similar dismutations.
2. Mechanism of antioxidant
Hydrogen is donation to free radicals by antioxidants and it forms of a complex between the lipid radical and the antioxidant radical (free radical acceptor).
R· + AH --> RH + A·
RO· + AH --> ROH + A·
ROO· + AH --> ROOH + A·
R· + A· --> RA
RO· + A· --> ROA
ROO· + A·--> ROOA
Antioxidant + O2--> Oxidized Antioxidant
3. Classification of antioxidants
According to the mechanism of antioxidants, antioxidant can be divided into:
(1) Free radical scavenger: mostly phenolic compounds like BHA,BHT,PG,TBHQ,tocopherols,spices extracts, flavonoids etc, they all have the role of electron donors
(2) Hydroperoxide decomposer: like sulfur-containing or selenium-containing compounds that decompose hydroperoxides to form non-radical products.
(3) Antioxidant synergist: like citric acid, malic acid, phosphoric acid, tartaric acid, lecithin, amino acid, ascorbic acid, etc.
(4) Singlet oxygen quencher: like Vitamin E, beta carotene etc.
(5) Lipoxygenase inhibitor: like glucose oxidase, superoxide dismutase, catalase, glutathione, peroxidase and so on.
(6) Metal Chelates: like EDTA, citric acid, phytic acid etc.