

The story of Artemisinin-a natural anti malarial drug

I. History
Artemisinin was isolated from sweet wormwood (Artemisia annua) leaves, a traditional Chinese remedy for fevers, in 1971. Its identification came out of a research project in
II. Anti-Malarial
Malaria is caused by the bite of a female mosquito (Anopheles), resulting in the malaria parasite, Plasmodium, entering the human blood stream. Once a red blood cell has become invaded with the parasite, several rounds of asexual reproduction ensue, leading to its eventual rupture. It is the cyclic release of parasites from the red blood cells which causes the intermittent symptoms of fever, shivering and anaemia that are characteristic of malaria. Of the four species of Plasmodium that infect humans, the most dangerous is P. falciparum. This parasite accumulates in the capillaries of vital organs such as the brain, kidney, intestine and lungs. Cerebral malaria, the accumulation of P. falciparum in the brain, is responsible for most of the deaths associated with malaria.

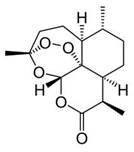
Artemisinin is one of the few effective treatments against falciparum malaria, The unusual structural feature of artemisinin, the 1,2,4-trioxane ring, is the basis for the unique antimalarial action of the drug. It is the endoperoxide linkage in the ring which 'triggers' artemisinin to 'explode' - but only in the vicinity of the Plasmodium parasite. The endoperoxide bond is cleaved when it comes into contact with iron(II), releasing reactive radicals which ultimately destroy the parasite. Substantial quantities of reactive iron(II) accumulate inside an infected red blood cell as a result of the liberation of the haem group, which is a byproduct of the digestion of haemoglobin. As Artemisinin is poorly soluble in water, more potent derivatives such as Dihydroartemesinin,Artemether,Arteether and Artesunate are developed.
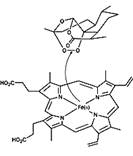
Cleavage of the endoperoxide bond in artemisinin by iron(II) in a haem group
The Artemisinin Derivatives
Artemisinin | ||
| Reduction | |
Dihydroartemisinin | ||
|
|
|
Ethyl Ether | Hemisuccinate | Methyl Ether |
Arteether | Artesunate | Artemether |
When treating both multidrug-resistant and non-drug resistant falciparum malaria, it is now recommended by WHO that artemisinin be taken in combination with another drug (this is a strategy designed to slow the development of resistance, since during treatment with two - or more - drugs, the chance of a mutant emerging which is resistant to both is drastically reduced). In the continuing absence of an effective malaria vaccine, the development of new antimalarial drugs - most likely derived from, or inspired by, artemisinin - will continue to be our primary weapon in the fight against malaria.
Main Artemisinin Combination Therapies or ACTs
1. Artemether/lumefantrine (AL)
2. Artesunate /amodiaquine(AS‐AQ)
3. Artesunate/mefloquine (AS-MQ)
4. Sulphadoxine/pyrimethamine(AS-SP)
5. Dihydroartemisinin/piperaquine (DHA-PPQ)
6. Artesunate/pyronaridine (AS-PY).
Malaria is UNITAID Supported Projects mainly purchased by following institutes:
1. Affordable Medicines Facility Malaria (AMFm)
2. A2S2
3. Global ACT demand forecasting
4. Malaria Diagnostics
III. Anti-Cancer
Cancer cells need irons to enable them to grow aggressively; hence cancer cells typically absorb a significantly larger amount of iron than normal, healthy cells. When Artemisinin come in contact with these irons in the cancer cells, it would trigger a chemical reaction and form charged atoms that chemists call "free radicals". The free radicals attack the cancer cell membranes, breaking them apart and killing them. This is why Artemisinin is highly toxic to cancer cells. Tests have been conducted to show that Artemisinin causes rapid and extensive damage and death in cancer cells and yet has relatively low toxicity to normal cells.
Studies are showing that Artemisinin has destructive activity against 55 cancer cell lines. Most notably Artemisinin has shown to be effective against cancer cells from leukemia, colon, lung, breasts to fibrosarcomas. It has also been shown that Artemisinin even killed cancers that were traditionally resistant to chemotherapy.