

Free Radicals
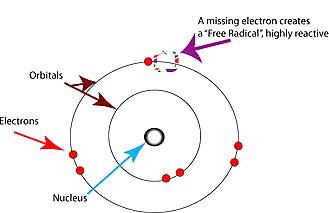
The human body is composed of cells. Cells are composed of molecules. Molecules consist of one or more atoms of one or more elements joined by chemical bonds. Atoms consist of a nucleus, neutrons, protons and electrons. The number of protons (positively charged particles) in the atom’s nucleus determines the number of electrons (negatively charged particles) surrounding the atom. Electrons are involved in chemical reactions and are the substance that bonds atoms together to form molecules. Electrons surround, or "orbit" an atom in one or more shells. The innermost shell is full when it has two electrons. When the first shell is full, electrons begin to fill the second shell. When the second shell has eight electrons, it is full, and so on.
The most important structural feature of an atom for determining its chemical behavior is the number of electrons in its outer shell. A substance that has a full outer shell tends not to enter in chemical reactions (an inert substance). Because atoms seek to reach a state of maximum stability, an atom will try to fill its outer shell by:
Gaining or losing electrons to either fill or empty its outer shell
Sharing its electrons by bonding together with other atoms in order to complete its outer shell
Atoms often complete their outer shells by sharing electrons with other atoms. By sharing electrons, the atoms are bound together and satisfy the conditions of maximum stability for the molecule.

Normally, bonds don’t split in a way that leaves a molecule with an odd, unpaired electron. But when weak bonds split, free radicals are formed. Free radicals are very unstable and react quickly with other compounds, trying to capture the needed electron to gain stability. Generally, free radicals attack the nearest stable molecule, "stealing" its electron. When the "attacked" molecule loses its electron, it becomes a free radical itself, beginning a chain reaction. Once the process is started, it can cascade, finally resulting in the disruption of a living cell.
Some free radicals arise normally during metabolism. Sometimes the body’s immune system’s cells purposefully create them to neutralize viruses and bacteria. However, environmental factors such as pollution, radiation, cigarette smoke and herbicides can also spawn free radicals.
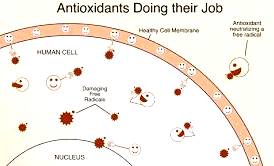
Normally, the body can handle free radicals, but if antioxidants are unavailable, or if the free-radical production becomes excessive, damage can occur. Of particular importance is that free radical damage accumulates with age.
Antioxidants neutralize free radicals by donating one of their own electrons, ending the electron-"stealing" reaction. The antioxidant nutrients themselves don’t become free radicals by donating an electron because they are stable in either form .They act as scavengers, helping to prevent cell and tissue damage that could lead to cellular damage and disease.
The antioxidants are believed to help protect the body from free-radical damage further to protect against cardiovascular and low the rates of cancer. The best way to ensure adequate intake of the antioxidant nutrients is through a balanced diet consisting of 5-8 servings of fruits and vegetables per day.